Tokyo R&D Facility
The U-Factor Tokyo R&D Facility is a key research hub for developing cutting-edge regenerative medicine technologies. Equipped with state-of-the-art facilities, including automated culturing and testing equipment, our clean facilities meet strict hygiene standards and are dedicated to the research of U-Factor® solution.


State-of-the-Art Clean Lab Combining Safety and Efficiency
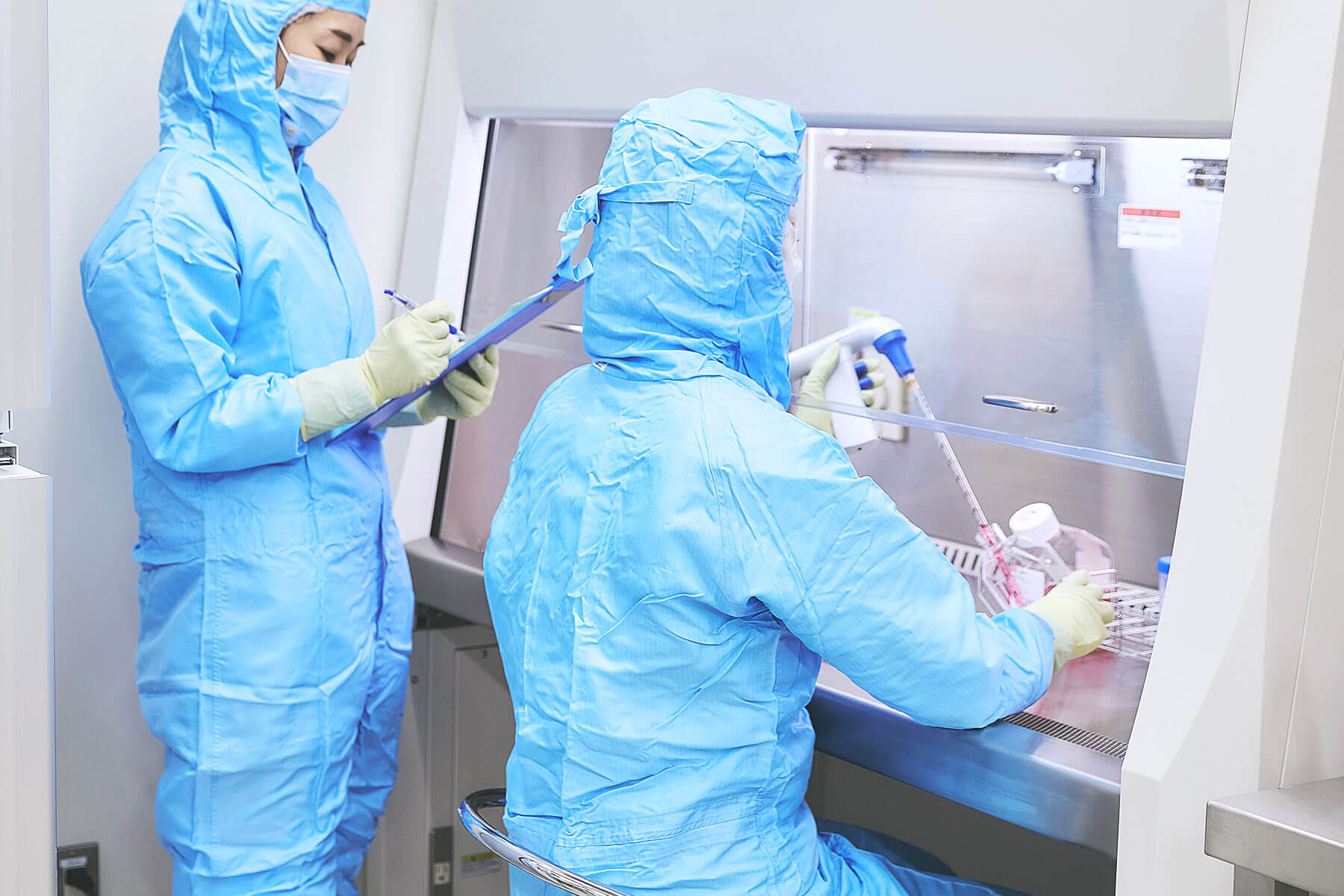
Our facility operates in compliance with GMP (Good Manufacturing Practice) standards and adheres to the stringent international guidelines set by ISO (International Organization for Standardization) and ICH (International Council for Harmonisation). Utilizing state-of-the-art equipment and advanced technologies, we conduct research that balances safety and efficiency. In our Grade B clean rooms, we maintain rigorous environmental controls to deliver high-quality research outcomes that address unmet medical needs and pioneer the future of medicine. Our researchers aim to provide practical solutions tailored to the needs of the medical field by employing innovative technologies.
Automation of Research Processes with Automated Culturing and Purification Equipment
At our research institute, we actively implement the latest automation technologies, including automated culturing and testing equipment. By eliminating quality variations caused by manual operations, we have significantly enhanced the consistency and efficiency of our research processes, reducing the likelihood of human error.
Automation has also enabled cost reductions, allowing us to allocate more resources to research and development. This approach enables us to consistently deliver high-quality research outcomes and provide affordable treatments to a larger number of patients, making a substantial contribution to the advancement of regenerative medicine.
- Achieving Uniformity and Consistency of U-Factor® Solution
- Achieving Cost Reduction through Automation
Conducting Cytokine Evaluation Tests In-House
At Tokyo R&D Facility, we conduct in-house evaluation tests of cytokines, which are the key components of the U-Factor® solution. We are working on formulating culture supernatants, a challenge that no one has attempted before.
We continue to take on the challenge of delivering an unprecedented therapeutic modality—stem cell culture supernatant—to patients suffering from intractable diseases.
Although the path is not always smooth, we will work as one with our collaborators and proceed with integrity, steadily establishing reliable quality and demonstrating safety and efficacy, thereby bringing a breakthrough therapy into real-world clinical use.
Atsutaka Noguchi
Director, Research & Development DepartmentDirector, Tokyo Research InstituteBorn in Hokkaido. After graduating from Hokkaido University, completed a master’s program at the Hokkaido University Graduate School. Joined our company in 2025 after working at Medinet Co., Ltd.

For those suffering from intractable diseases, a better U-Factor® solution.
U-Factor® solution's research and development is supported by a talented team with diverse expertise. Our researchers, facility staff, and external experts work together, combining innovative technology and knowledge to advance our studies. Each team member leverages their strengths and prioritizes teamwork to achieve high-quality research outcomes.
We value communication and collaboration, tackling problems to establish innovative treatments that meet patient needs. Under the motto "Creating a Better U-Factor® Solution," professionals from various fields and positions, including researchers, culturing staff, and external experts, gather at the Tokyo Research Institute to drive the R&D of the U-Factor® solution forward.