NEWS News Release
Announcement of the Commencement of a Specific Clinical Study for ALS
Announcement of the Commencement of a Specific Clinical Study for ALS
-Towards ALS Treatment Using Deciduous Tooth Pulp Stem Cell Culture Supernatant “U-Factor® Solution”-
U-Factor Co., Ltd. (Headquarters: Chiyoda-ku, Tokyo; CEO: Hidehiro Ijima, hereinafter referred to as “U-Factor”) and Hitonowa Medical (Headquarters: Chiyoda-ku, Tokyo; Director: Yasuhiro Seta) have jointly commenced a specific clinical study targeting ALS (Amyotrophic Lateral Sclerosis) patients in March 2024. This study will involve the administration of “U-Factor® Solution,” a deciduous tooth pulp stem cell culture supernatant developed by U-Factor, across multiple medical institutions to assess its safety and efficacy in treating ALS.


1. Background
ALS is a disease where the muscles in the limbs, throat, tongue, and those necessary for breathing gradually weaken and lose function. This condition primarily affects motor neurons, leading to the loss of communication between the brain and muscles. While sensory functions, vision, hearing, and internal organs remain unaffected, ALS progresses relentlessly, often leading to death within 3 to 5 years after onset. It is estimated that there are approximately 456,000 ALS patients worldwide, with about 10,000 in Japan (according to the Japan ALS Association). Every year, between 1,000 and 2,000 new cases are diagnosed in Japan. With no effective treatment available, ALS is designated as an intractable disease, representing a significant unmet medical need. The ALS Ice Bucket Challenge, a global phenomenon around 2014, brought considerable attention to this disease. Before launching this specific clinical study, a retrospective study conducted at Hitonowa Medical demonstrated the safety and efficacy of U-Factor® Solution, leading to the decision to proceed with this clinical study.
2. Overview of the Specific Clinical Study
This clinical study will evaluate the safety and efficacy of the investigational drug “U-Factor® Solution” in ALS patients.
| Study Protocol Number | jRCTs031230731 |
|---|---|
| Study Title | Evaluation of the Safety and Efficacy of Deciduous Tooth Pulp Stem Cell Culture Supernatant for ALS |
| Principal Investigator | Yasuhiro Seta |
| Study Period | 2024-03-25 – 2025-12-31 |
| Target Population | ALS patients |
| Study Sites | Hitonowa Medical and multiple other medical institutions |
| Investigational Drug | U-Factor® Solution |
3. What is Deciduous Tooth Pulp Stem Cell Culture Supernatant “U-Factor® Solution”?
U-Factor® Solution is a highly purified supernatant obtained through our proprietary technology during the cultivation of stem cells. It contains a large quantity of cytokines (proteins with physiological effects) secreted by the stem cells. Its efficacy was first discovered by U-Factor’s Director and Professor Emeritus Minoru Ueda of Nagoya University Graduate School of Medicine and colleagues (M Ueda, et al. Neurosci 2015). Through further refinement by our company, U-Factor® Solution has shown significant potential in terms of efficacy. 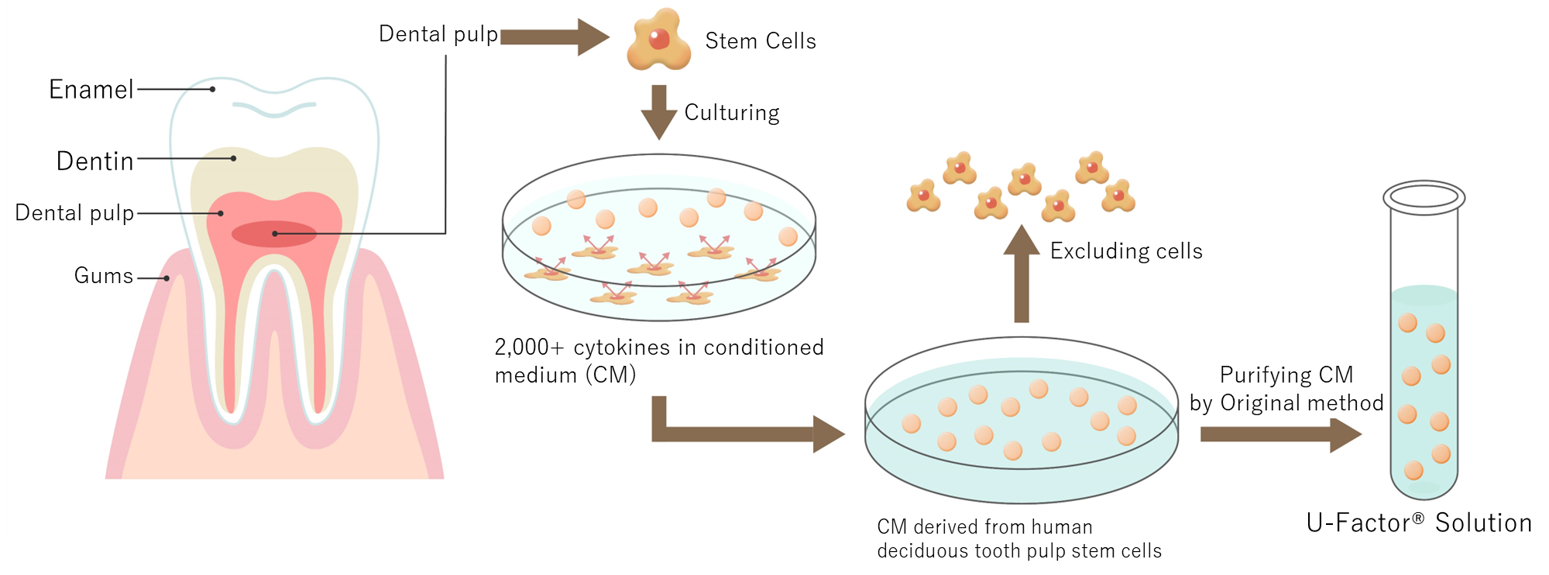
Comments from the Representatives
I am confident that U-Factor® Solution, which has been developed through extensive research, will meet unmet medical needs. As the first step, we are initiating a specific clinical study targeting ALS patients. By maximizing “quality” and ensuring “quantity” through the development of automated culture systems, we are committed to making U-Factor® Solution a beacon of hope for ALS patients through this clinical study.

We are undertaking clinical research on ALS with the aim of sharing our findings with the world, providing new treatment options for patients suffering from intractable diseases, and contributing to the advancement of medicine. As a medical institution that walks alongside our patients, Hitonowa Medical is dedicated to furthering the field of regenerative medicine and making a global impact through both research and treatment.

Established: March 2020
CEO: Hidehiro Ijima
Headquarters: Mitsui Link Lab Kasai, Room 205, Kasai Research & Development Center, Daiichi Sankyo Co., Ltd., 1-16-13 Kitakasai, Edogawa-ku, Tokyo
URL: https://u-factor.com
Business: U-Factor is focused on developing therapeutic drugs for intractable diseases such as Alzheimer’s disease using U-Factor® Solution. Through joint research and development with institutions such as the National Institute of Advanced Industrial Science and Technology (AIST) and Keio University School of Medicine, we are working on the development of new drugs to address various unmet medical needs such as Alzheimer’s disease and ALS. There are many diseases for which effective treatments have not yet been established, and we are committed to validating and proving the safety and efficacy of the supernatant we have developed for such conditions.
Established: October 2020
Director: Yasuhiro Seta
Headquarters: 2nd Floor, K. Plaza Building, 1-7 Rokubancho, Chiyoda-ku, Tokyo
URL: https://u-factor-hitonowa.com
Philosophy: Hitonowa Medical is a medical institution dedicated to providing care that “stands by the patient and faces the disease together,” focusing on “regenerative medicine x intractable diseases.” Since its opening in 2020, the clinic has been treating ALS, stroke, and dementia using stem cell culture supernatant. With the aim of bringing together patients, their families, and healthcare professionals to face these diseases, Hitonowa Medical continues to contribute to both research and treatment in the field of regenerative medicine.
U-Factor Co., Ltd.
Phone: +81-3-5357-1580
Email: info@u-factor.com
Hitonowa Medical
Phone: +81-3-6272-8181
Email: info@u-factor-hitonowa.com