

Clinical Research ALS with Hitonowa Medical
This specific clinical study evaluates the safety and efficacy of the U-Factor® solution in patients with ALS.
- Study Title
- Evaluation of the Safety and Efficacy of stem cells from human exfoliated deciduous teeth Culture Supernatant Administration for ALS
- Protocol Number
- jRCTs031230731
- Study Duration
- March 25, 2024 - December 31, 2025
- Participants
- ALS Patients
- Research Facility
- Hitonowa Medical
- Principal Researcher
- Yasuhiro Seta
- Investigational Drug
- U-Factor® solution
Evaluation of the Safety and Efficacy of U-Factor® Solution Administration
As a preliminary phase to this specific clinical study, a retrospective study was conducted at Hitonowa Medical on the administration of U-Factor's stem cells from human exfoliated deciduous teeth Culture Supernatant, U-Factor® solution. The study demonstrated favorable results in terms of safety and efficacy. Consequently, we have planned and initiated this specific clinical study.
About ALS
ALS is a disease in which the muscles of the limbs, throat, tongue, and those needed for breathing gradually weaken and atrophy, leading to loss of function. The primary impact is on motor neurons, resulting in a disruption of commands from the brain to move the limbs. Meanwhile, sensations, vision, hearing, and internal organ functions are generally preserved. ALS is a severe disease with no halt in progression post-onset, typically leading to death within 3 to 5 years.
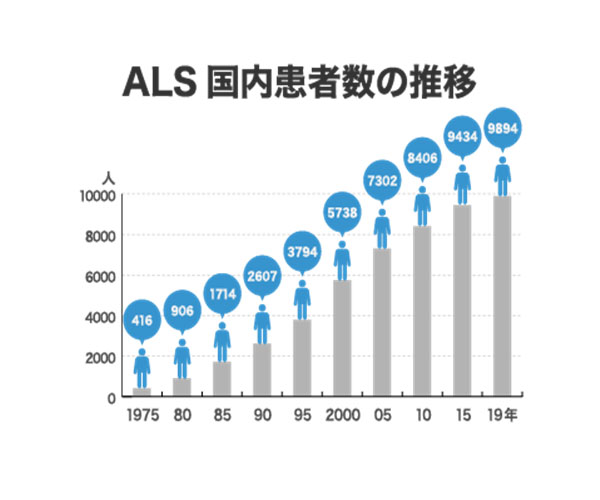
Globally, the estimated number of ALS patients is about 456,000, with approximately 10,000 in Japan (according to the Japan ALS Association). Each year, 1,000 to 2,000 new cases are diagnosed in Japan. With no effective treatments available, ALS is designated as an intractable disease and is considered a prime example of unmet medical needs, where effective therapies are still lacking.
In 2014, ALS gained significant attention as the subject of the Ice Bucket Challenge, a social media phenomenon where participants poured ice water over their heads or donated to ALS research, raising awareness and funds for the disease.
We provide U-Factor® solution to Hitonowa Medical and are engaged in a specific clinical study for ALS.
We are conducting a clinical study on ALS and aim to share our findings with the world to provide new treatment options for patients suffering from intractable diseases, thereby contributing to the advancement of medicine. As a medical institution that walks alongside patients, Hitonowa Medical is committed to contributing globally in both research and treatment while striving for further developments in the field of regenerative medicine.
Director of Hitonowa Medical
Yasuhiro Seta


Hitonowa Medical
- Establishment
- October 2020
- Address
- 1-7 Rokubancho, Chiyoda-ku, Tokyo, K. PLAZA 2nd Floor
- TEL
- +81 3-6272-8181
- Philosophy
- Hitonowa Medical is dedicated to providing medical care centered on "Regenerative Medicine x Intractable Diseases," with a commitment to "supporting patients and facing illnesses together." Since its opening in October 2020, we have been utilizing stem cell-derived culture supernatants to treat intractable diseases such as ALS, stroke sequelae, and dementia. By harnessing the power of regenerative medicine, we aim to create a cohesive circle involving patients, their families, and healthcare professionals, working collaboratively to confront and manage these challenging conditions.